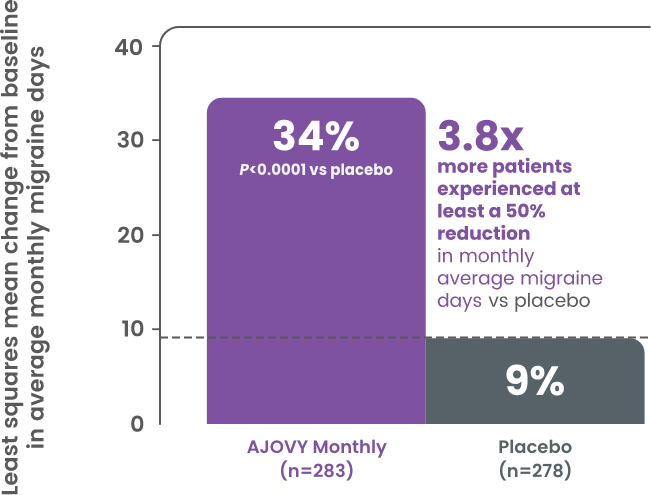
Results in patients with an inadequate response to anticonvulsants or neurotoxins (exploratory analysis)‡
- 3.8 mean reduction from baseline in monthly average migraine days for both monthly and quarterly arms vs 1.0 for placebo in patients with previous anticonvulsant use2
- 2.7 and 3.2 mean reduction from baseline in monthly average migraine days for both monthly and quarterly dosing arms, respectively, vs 0.2 for placebo in patients who previously used neurotoxins2
‡For all exploratory analyses, no determination of statistical significance can be made and no conclusions should be drawn.
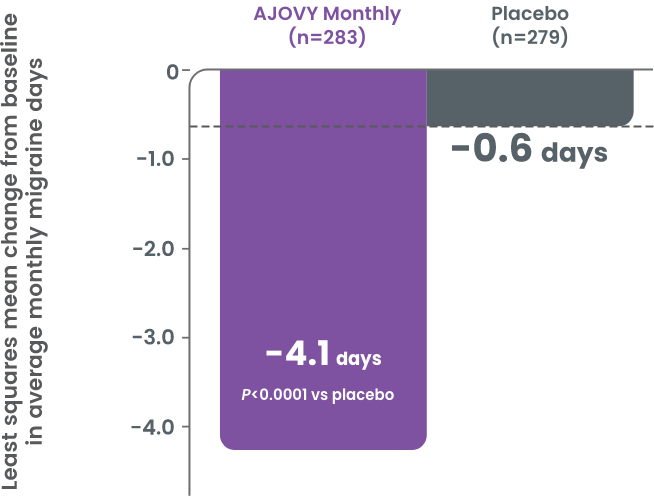
Patients experienced a significant reduction in the monthly average number of days using migraine-specific acute headache medication (secondary endpoint)2
- Mean reduction of 3.9 days for monthly dosing and 3.7 days for quarterly dosing of migraine-specific acute headache medication use vs 0.6 days with placebo2