Eosinophil Reductions
CINQAIR was proven to reduce blood eosinophil counts compared with placebo.
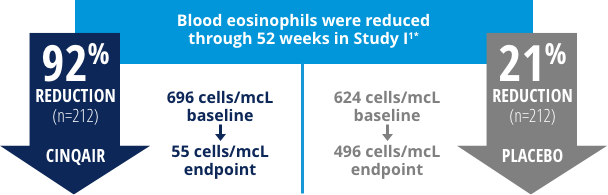
Results were similar in Study II.2
A reduction in blood eosinophil counts was seen 2-3 days after the first dose3
In a subset of patients from Study I and Study II receiving CINQAIR (n=35) and placebo (n=32), eosinophil counts were reduced:

Reductions in blood eosinophil counts were related to serum levels of CINQAIR.1
Study Design: Studies I-II1,2
Design: 52-week studies comparing treatment with CINQAIR or placebo along with background asthma therapy in 953 patients with severe asthma who were required to have a blood eosinophil count ≥400/mcL (within 3-4 weeks of dosing) and ≥1 asthma exacerbation requiring systemic corticosteroid use over the past 12 months. The majority of patients were on medium-high dose inhaled corticosteroids (ICSs) plus a long-acting beta agonist (LABA) at baseline. Maintenance OCSs were allowed.
Primary Endpoint: Frequency of asthma exacerbations.
Secondary Endpoints: Change from baseline in forced expiratory volume in one second (FEV1), blood eosinophil count, Asthma Control Questionnaire-7 (ACQ-7) score, and Asthma Quality of Life Questionnaire (AQLQ) total score.
Exacerbation Definition: A worsening of asthma that required at least 1 of the following medical interventions:
- Either the use of a systemic corticosteroid (if not already taking), or a ≥2-fold increase in the use of ICSs for 3 or more days, and/or
- Asthma-related emergency treatment including at least 1 of the following: an unscheduled visit to their healthcare professional for nebulizer treatment or other urgent treatment to prevent worsening of asthma symptoms; a visit to the emergency room for asthma-related treatment; or an asthma-related hospitalization
The mechanism of CINQAIR action in asthma has not been definitively established.1
* Study design included 2 identical, double-blind, parallel-group, randomized, placebo-controlled trials. The average reduction in blood eosinophils was measured through 52 weeks.4
REFERENCES: 1. CINQAIR Prescribing Information. West Chester, PA. Teva Respiratory, LLC. 2. Data on file (clinical study report: a 12-month, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of reslizumab [3.0 mg/kg] in the reduction of clinical asthma exacerbations in patients [12-75 years of age] with eosinophilic asthma. Study C38072/3083). Parsippany, NJ. Teva Respiratory, LLC. February 2015. 3. Data on file (clinical study report: a 12-month, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of reslizumab [3.0 mg/kg] in the reduction of clinical asthma exacerbations in patients [12-75 years of age] with eosinophilic asthma. Study C38072/3082). Parsippany, NJ. Teva Respiratory, LLC. January 2015. 4. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355-366.